Have you heard about CIP and SIP? What are the differences between CIP and SIP? Let’s figure out in this article.
Contents
Introduction
Equipment sterility and hygiene maintenance are essential for product quality, consumer safety, and regulatory compliance. Automated Cleaning-in-place (CIP) and Sterilization-in-place (SIP) systems are created to achieve these goals. They help clean and sterilize the equipment without disassembling or removing it. In this article, let’s explore the CIP and SIP systems and their differences.
Definition and purposes
Cleaning-in-place (CIP) is an automatic cleaning method that removes impurities and residues in plant equipment without disassembling or removing it. The equipment, including pipes, machines, and tanks, is intact while cleaning solutions pass through it like the products. The common CIP detergents combine water, heat, and chemicals.
SIP usually takes place after CIP and is the final step in the cleaning procedure. SIP stands for sterilization-in-place or steam-in-place. This process aims to automatically clean and sterilize equipment and ensure a sanitized environment by using steam to kill off any microorganisms. In addition, other SIP cleaning techniques include superheated water, gas, liquid, dry heat, and vapor sterilization. During the SIP process, the equipment is also kept intact.

Explore more about CIP in the beverage industry:
Advantages of CIP and SIP systems
CIP and SIP systems offer several similar advantages to processing operations. Applying CIP and SIP processes can help increase sterility levels, product safety, and quality and lower the risk of contamination. Another significant advantage is their automatic and fast cleaning, reducing the errors caused by manual methods. Additionally, manufacturers can the cleaning results can be controlled, repeatable, and validated. These systems help lower downtime and make production far more efficient. People clean the equipment without removing or disassembling it, saving time and reducing damage.
Besides, automated CIP and SIP processes can help lessen employee chemical exposure and save the labor force for other tasks as the equipment stays intact during cleaning and the processes are automated. Water and other detergents can also be reduced and reused to minimize operation costs and environmental impacts. The combination of CIP and SIP processes provides advanced and thorough cleaning and increases production effectiveness.

CIP and SIP processes
CIP process
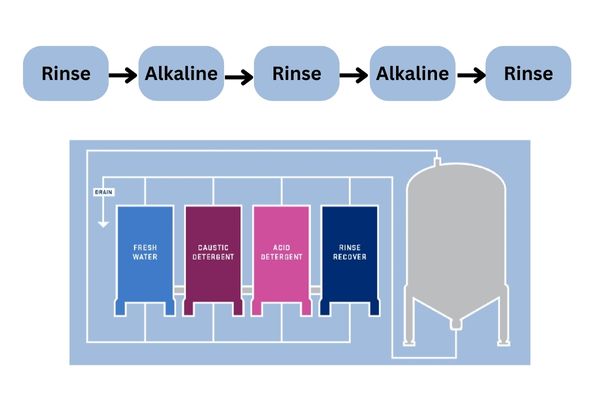
CIP methods apply mechanical, chemical, and thermal forces to clean the equipment. Some commonly used chemicals are sodium hydroxide, phosphoric, and nitric acids. In general, a typical CIP cycle can take 60 to 90 minutes, with five steps, including pre-rinse, alkaline cleaning, rinse, acid cleaning, and final rinse.
- Firstly, water flow removes the residues of products and dissolves the leftover sugars and fats.
- Next, use an alkaline detergent to clear away organic soil in pipes and tanks, while controlling the temperature at around 60-70°C.
- After that, clean water will remove detergent traces and dissolved residues.
- An acid wash is then applied to take off the minerals and leftover proteins.
- For the final rinse, use RO water to ensure that all cleaning detergents and spoil are removed.
SIP process
To start a SIP process, the system must be clean first, which is why SIP often follows after the CIP cycle. A typical SIP process includes three steps: heating up, holding, and cooling down.
- In the first step, sterilants, such as saturated steam, hot water, or chemical sterilants, are fed into the system and heated to about 120 – 135°C, much higher than the temperature of the CIP process.
- During the holding stage, the sterilant is maintained in the system for a specific time, from 15 to 40 minutes, while the pressure and temperature are strictly controlled. Too long exposure can negatively impact the gaskets, hoses, and other equipment components.
- After that, cool down and dry the system before production.
Structures of CIP and SIP system
Depending on the specific products and factory sizes, the structure of CIP and SIP systems can be different. However, they usually contain some common components.
Tanks: Tanks are the main components of the cleaning system and are often made from stainless steel. In the CIP process, tanks contain water and alkaline or acid solutions. Depending on the purposes and product types, there may be single or multiple tanks, usually up to four. The number of tanks is based on the cleaning detergents used. In general, three and four-tank systems are more advanced and save more time for the cleaning process. Meanwhile, the SIP process has gas tanks.

Pumps: In the CIP process, the supply pump offers the necessary pressure to generate a turbulent flow to all equipment’s components. Meanwhile, the return pump aims to return or drain the used cleaning solutions. In addition, the metering and dosing pump help provide chemicals automatically and precisely to the machines. While in the SIP system, pumps are often used to circulate heated water or steam-condensate mixes to maintain consistent sterilization.
Spraying devices: The spraying devices play an important role in the tank cleaning cycles. They help clean the entire interior surface of the tanks without filling them with detergents, saving a significant amount of cleaning solutions and steam. Some popular spraying devices are static spray balls, rotating spray balls, and rotary jet heads. The spraying devices are essential for both CIP and SIP processes.
Heating devices: Because the appropriate temperature is crucial in both systems, the heating devices are necessary to control the temperature.
Pipes: Both systems contain distribution piping to transfer cleaning solutions and steam. They are often equipped with valves to control them.
Sensors: The system needs temperature and pressure sensors to ensure the cleaning process and sterilization meet the requirements.
In addition, the SIP system has boilers or steam generators to generate sterilizing steam.
Applications of CIP and SIP processes
Cleaning-in-place and Sterilization-in-place processes are essential to guarantee production efficiency, safety, and quality of products. Ensuring hygiene standards during the production process is also one of the criteria for achieving many certifications, such as FSSC and ISO. Therefore, CIP and SIP processes are applied in industries requiring a high level of sterility and cleanliness. These two processes often go together to double the cleaning and sterilization.
Well-established CIP systems can be found wherever the strict sanitary regulations are followed. The fast and efficient CIP process is crucial in the food and beverage and brewer industries to remove all the residues and bacterial risks after each production cycle. Dairy products are especially susceptible to bacterial growth and spoiling, so CIP is inevitable. Besides, the pharmaceutical industry requires a high level of CIP and SIP technology to ensure the most stringent hygiene requirements.

Conclusion
With outstanding advantages, CIP and SIP systems are crucial for modern manufacturing plants, especially in the food and beverage, brewer, and pharmaceutical industries. These two methods can help ensure product quality and customer safety while optimizing operation costs and increasing efficiency. The applications of CIP and SIP processes are crucial in promoting long-term success.
About Future Generation Co.,Ltd
We are FGC, the leading tea and beverage exporter in Vietnam and one of the biggest tea suppliers in the world. Our tea products are diverse in types, grades, and production methods. We provide customers with Loose tea (such as Black tea, Green tea, and Oolong tea), Herbal tea (such as Rose tea, Chamomile tea, and Lotus tea), and Specialty tea (such as Matcha), RTD Tea, and Tea Bags. Our mission is to become Vietnam’s leading healthy beverage company.
We ensure stable tea sources for domestic markets and exports with vast high-grown tea farms and available herb gardens. Our tea gardens and factories also meet international standards such as ISO, HACCP, KOSHER, HALAL, etc. In addition, we constantly innovate our machinery system, strengthen production capacity, and increase productivity. We also provide private label and customized packaging services for small and medium enterprises.
If you are a tea distributor, tea importer, teashop manager, or even tea lover, we are committed to being your prestigious tea source supplier in Vietnam!
Contact
Address: R4 building, Office Quarter 02, Royal City, 72A Nguyen Trai St., Thanh Xuan Ward, Hanoi.
Phone: +84 24 73 000 125/ +84 24 73 063 369
Mail: info@vietnam-tea.com
Website: https://vietnam-tea.com/
Facebook: https://www.facebook.com/fgcvietnamtea










